
What are the building blocks of molecules?
This section will teach us how to:
- Describe what matter and elements are
- Describe the relationship between electrons, protons, & neutrons, as well as the different ways which electrons are shared between certain atoms
Matter, is essentially the material which makes up life. Matter, is that which has mass and occupies some space. All matter is made up of elements. Those elements are made up of a certain amount of atoms. Those atoms have a constant number of unique properties as well as protons (discussed below). There are 118 elements, though only 92 of them occur naturally. Of those 92 that occurs naturally, there are fewer than 30 which are located in living cells. There is 26 which are not stable and don’t exist for long or they are considered theoretical and have not been detected.
Every element has its own chemical symbol (some of these are H, O, C, N, etc). They each also have unique properties which make them up. These properties all different elements to combine and form chemical bonds with each other.
The Atom
The smallest component of an element, which still retains its chemical properties, is considered an atom. A hydrogen atom, for example, has all the unique properties of the hydrogen element. Properties such as existing in the form of gas when at room temperature. Or the fact that it can bond with oxygen and creates the water molecule. Once the hydrogen atom is broken into subatomic particles, it wouldn’t have hydrogen’s properties anymore.
Living organism are, at their most basic level, made up of a combination of elements. They have atoms which combine together and form molecules. In any multicellular organism (for example, animals), molecules interact to form the cells, which then combine and form tissue, which then makes organs. These different combinations happen until an entire multi-celled organism is formed.
The Makeup of the Atom
All atoms are made up of protons, neutrons, and electrons. Only hydrogen (H), is made up of one electron and one proton. An electron is a particle with a negative charge and travels around the core of the atom (or nucleus). The proton has a positive charge (or +1) and the neutron has no charge. Both the proton and the neutron reside in the nucleus. The proton and neutron have a mass of 1. The mass of the electron is negligible with a negative charge or (-1). The electron travels outside of the nucleus.
Neutral atoms have a net zero charge. This means the protons(positive) and electrons(negative) end up balancing each other out.
Since both neutrons and protons have a mass of 1, the mass of the atom would be equal to the number of neutrons and protons that atom has. Since the mass of an electron is so small and negligible, we do not need to factor in its overall mass.
As was mentioned earlier, every element has unique properties. This means they each have different numbers of neutrons and protons which gives them each their own mass number and atomic number. A mass number is also referred to as atomic mass. This is the sum of the protons and the neutrons of that element. The atomic number is the number of protons which that element contains. We can find the number of neutrons in an element when we subtract the atomic number from the mass number.
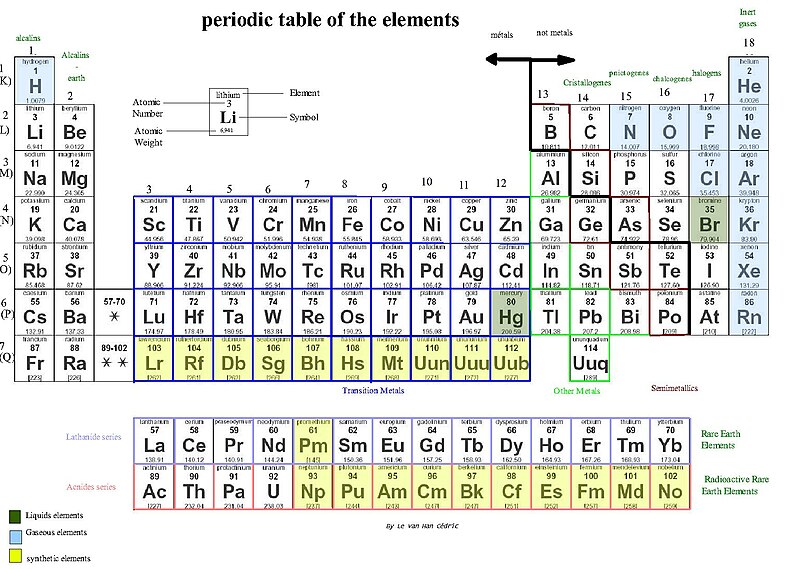
The mass number and atomic number are both useful and provide info about the different elements as well as how they will interact and react when combined. Certain elements have certain boiling and melting points. They are in different states (gas, solid, or liquid) at different temperatures. Different elements combine in different ways. Some elements will form specific types of chemical bonds, while others will not. This is based on how many electrons are present. Because of this, the different elements are placed into the periodic table of elements. This is a chart of the different elements which includes their atomic number as well as the relative atomic mass of each number. The table of elements also provides important info about the properties of the elements (usually available through color-coding).
One can get different forms of the same element. These are called isotopes. Isotopes are elements with the same number of protons but have a different number of neutrons. There are some naturally occurring isotopes, such as uranium, carbon, and potassium. Carbon-12 is the most common isotope of the element carbon. This element contains six protons and six neutrons. For this reason, it has a mass number of 12 (6 protons and 6 neutrons) and the atomic number of 6(which makes it the element carbon). Then there is carbon-14 which has eight neutrons and six protons. For this reason, it has a mass number of 14 with an atomic number of 6. Both of these forms of carbon are isotopes of the carbon element. There are isotopes which are unstable and lose subatomic particles, protons, or energy which allows them to become stable. Those elements are referred to as radioactive isotopes (also called radioisotopes).
Chemical Bonds
Elements have the ability to interact with each other. This depends on how exactly the electrons are arranged. The number of openings for electrons which exist in the outermost region of the element. In an element, the electrons don’t exist in the nucleus, but rather live at different energy levels that form around the nucleus. The closest shell holds up to 2 electrons. The elements closest shell is always filled first. Since Hydrogen has one electron, it only occupies the lowest shell. Then there is Helium, which has 2 electrons and completely fills the lowest shell. The periodic table above separates the rows by how many levels that row has. The first row only has 1 energy level, then the 2nd row has 2 energy levels and so on.
The next levels (level 2 and 3) can hold up to 8 electrons each. Those electrons are in four pairs and one of the positions in those pairs is always filled before any pair is completed. When we look at the 2nd row, we see lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. All of these elements have electrons which occupy the first and second shells. The first element of the second row, Lithium, only has one electron in its outermost shell, and then beryllium has two, and so on until 8 electrons fill the 2nd energy level (neon).
Elements are most stable when all of the electron positions in the outermost shell is filled. When there is a vacancy in the outermost shell, the possibility of a chemical bond increases. A chemical bond is an interaction between 2 or more of different or the same elements which results in the formation of molecules.
In pursuit of greater stability, we see atoms tend to totally fill their outer shells and will bond with other elements to accomplish their goal. They do this by sharing electrons, donating electrons to other atoms, or accepting electrons from another atom.
Elements with low atomic numbers (this goes up to calcium which has an atomic number of 20), can hold up to 8 electrons in their outer shell. This is referred to as the octet rule. An element will donate, hare, or accept electrons to fill the outer shell and satisfy the octet rule.
If an atom doesn’t have an equal number of electrons and protons, we call it an ion. When the number of protons and electrons is not equal, each ion produces a net charge. If we lose electrons, we have a positive ion, also called a cation. If we gain an electron (in other words produce a negative ion) we call those anions.
Let’s take sodium under consideration. Sodium has one electron in its outermost shell. It takes a lot less energy for that sodium element to donate one electron that for it to accept seven more electrons to fill its outer shell. If it was to lose its electron, it would now have 11 protons and 10 electrons. This gives it an overall charge of +1. This is now a sodium ion.
Then there is the chlorine atom. This has seven electrons in its outer shell. This means it is easier for it to gain 1 electron than to lose 7. For this reason, chlorine tends to gain an electron and creates an ion with 18 electrons and 17 protons. This gives it a net negative charge (-1). This would now be considered a chlorine ion. The movement of an electron from an element to another is called an electron transfer.
If we had sodium and chloride it would be likely that since sodium(NA) only has in its outer shell 1 electron, and chlorine(Cl) has 7 electrons in its outermost shell, this means the sodium element will give its electron to empty its shell. This produces 2 ions. A sodium ion with a +1 ionic charge, or cation, and chlorine becomes an anion ion with -1 charge.
Different Types of Chemical Bonds
The four types of bonds (also called interactions): Ionic, covalent, hydrogen, van der Waals.
Ionic Bonds
A positive ion is formed when an element donates its electron from its outer shell (like in our sodium example). Whichever element accepts the electron suddenly becomes negatively charged. Since negative and positive charges attract, those ions stay together and will form ionic bonds, which is a bond between ions. With Sodium (which has 1 extra electron on its outer shell) and Chlorine (which has 7 electrons in its outer shell), sodium will lose an electron which gets transferred to the chlorines outer shell which is in need of an electron. They do this because atoms seek to be stable, and this can happen when the outer shell is filled! These elements will bond together with the electron from one of the elements staying predominantly with the other element. When the positive sodium ion (NA+) and negative chlorine (Cl-) ions mix and combine and produce NaCl, the electron from the sodium atom stays with the other 7 that are from the chlorine atom. The chloride and sodium ions attract each other with a net zero charge.
Covalent Bonds
A covalent bond happens whenever electrons are shared between 2 elements. These are the strongest and also the most common types of chemical bonds in living organisms. The elements which make up biological molecules in cells, happen because of covalent bonds. While covalent bonds do not dissociate in water, ionic bonds do.
The oxygen and hydrogen atoms which combine to form the water molecule are bound together as a covalent bond. The electron which is in the hydrogen atom will divide its time between its outer shell and the incomplete outer shell of the oxygen element. In order to fill its outer shell, the oxygen atom uses 2 electrons from the hydrogen atom (that’s why subscript “2” in H2O). The atoms share the electrons. They divide there time to share the time spent between them to “fill” those outer shells of each. This sharing is a low energy state compared than if they existed with no outer shells filled.
2 Types of Covalent Bonds
The 2 types of covalent bonds are polar and nonpolar.
In nonpolar covalent bonds, 2 atoms form, of the same element and share their electrons equally. An example of this is found when we look at the oxygen atom. An oxygen atom can bond itself with another atom and they will fill their outer shells. This is because this bond requires 2 shared electrons in order to fill the other most shell of both. Then there is the example of nitrogen which can form 3 covalent bonds (these are also referred to as triple covalent bonds) between 2 atoms of nitrogen since each atom requires up to 3 electrons to fill its outer most shell. Finally, we can look at CH₄ (methane). This molecule. Usually, a carbon atom will have 4 atoms in its most outermost shell. This, of course, means it needs 4 more electrons to stabilize. Once it gets 4 from hydrogen, its a wrap… we have methane. These 4 electrons are provided by four hydrogen atoms, which each provides 1 electron. They all share their electrons equally. This creates four nonpolar covalent bonds.
Then, in a polar covalent bond, electrons which are shared by the atom spend more time closer to a specific nucleus than the other nucleus. Because there is an unequal distribution of electrons between the different nuclei, different slightly negative, or slightly positive charge develops (δ–, (δ+)). There is a covalent bond between the oxygen and hydrogen atoms of water. These are polar covalent bonds. They share electrons and spend more time near the oxygen nucleus. This gives it (the oxygen atom) a negative charge (albeit a small one), and the time it spends in the hydrogen atom, give the hydrogen atom a positive charge.
Hydrogen Bonds
The first thing to say about hydrogen bonds is that they are weaker than ionic and covalent bonds! This is because it takes less energy to break these type of bonds.
Hydrogen bonds are intermolecular bonds, which means they form between molecules (Between 2 different molecules), as opposed to ionic and covalent bonds which happen between atoms.
When there is a polar covalent bond which contains a hydrogen atom, the hydrogen atom that bonds, has a slightly positive charge. This is of course because of the shared electron pulling strongly toward the other element and away from the hydrogen nucleus. Since the hydrogen is slightly positive, this will attract it to the slightly negative charge of nearby molecules.
Van der Waals Interactions
Very similar to hydrogen bonds, van der Waals interactions are also weak interactions or attractions that happen between molecules. These will occur between covalently bound, polar atoms in different molecules. Weak attractions which are caused by temporary small changes which form when electrons are moving around a nucleus cause these bonds.